- Details
- Written by Suzana Häggi
- Hits: 369

- Information
- Subscribe
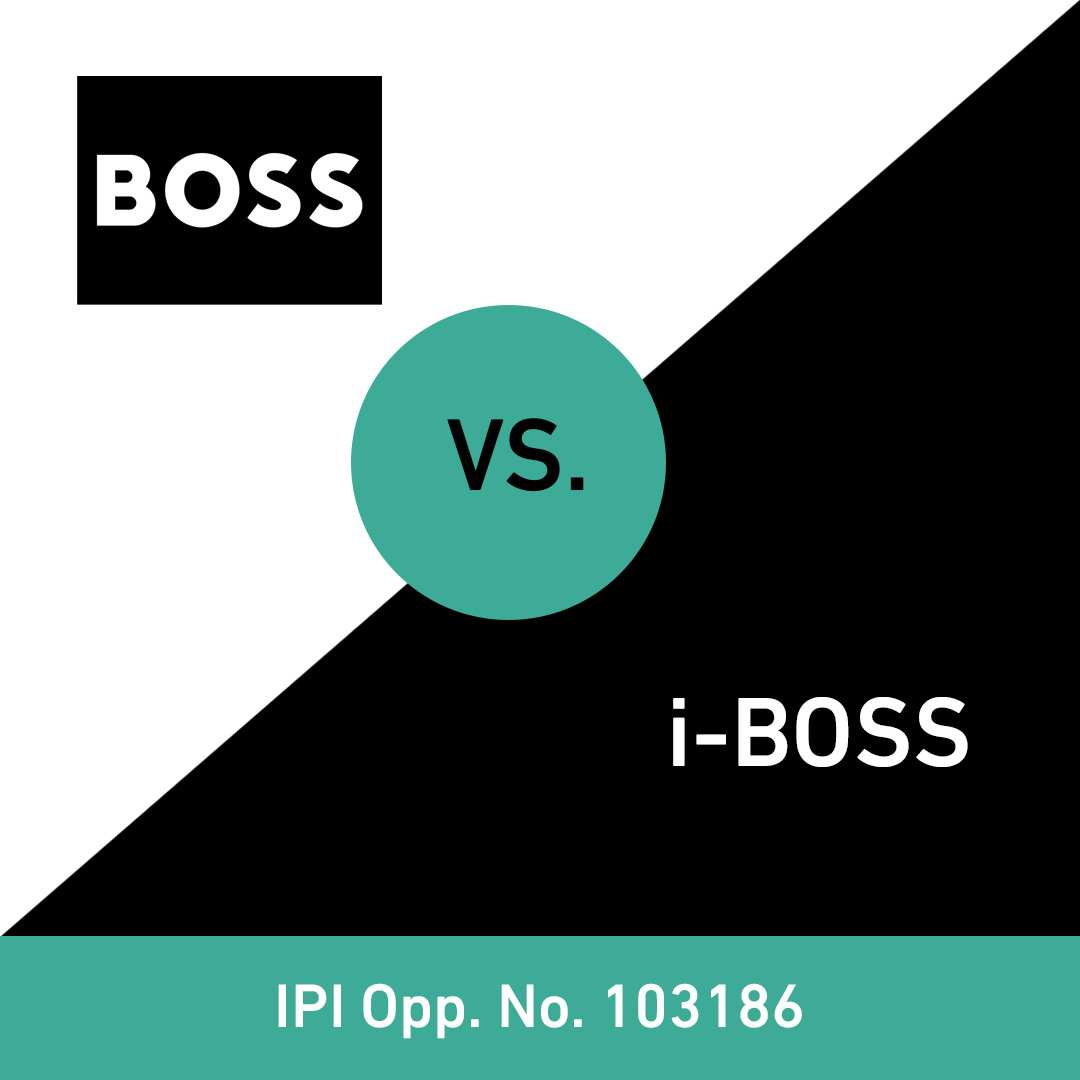
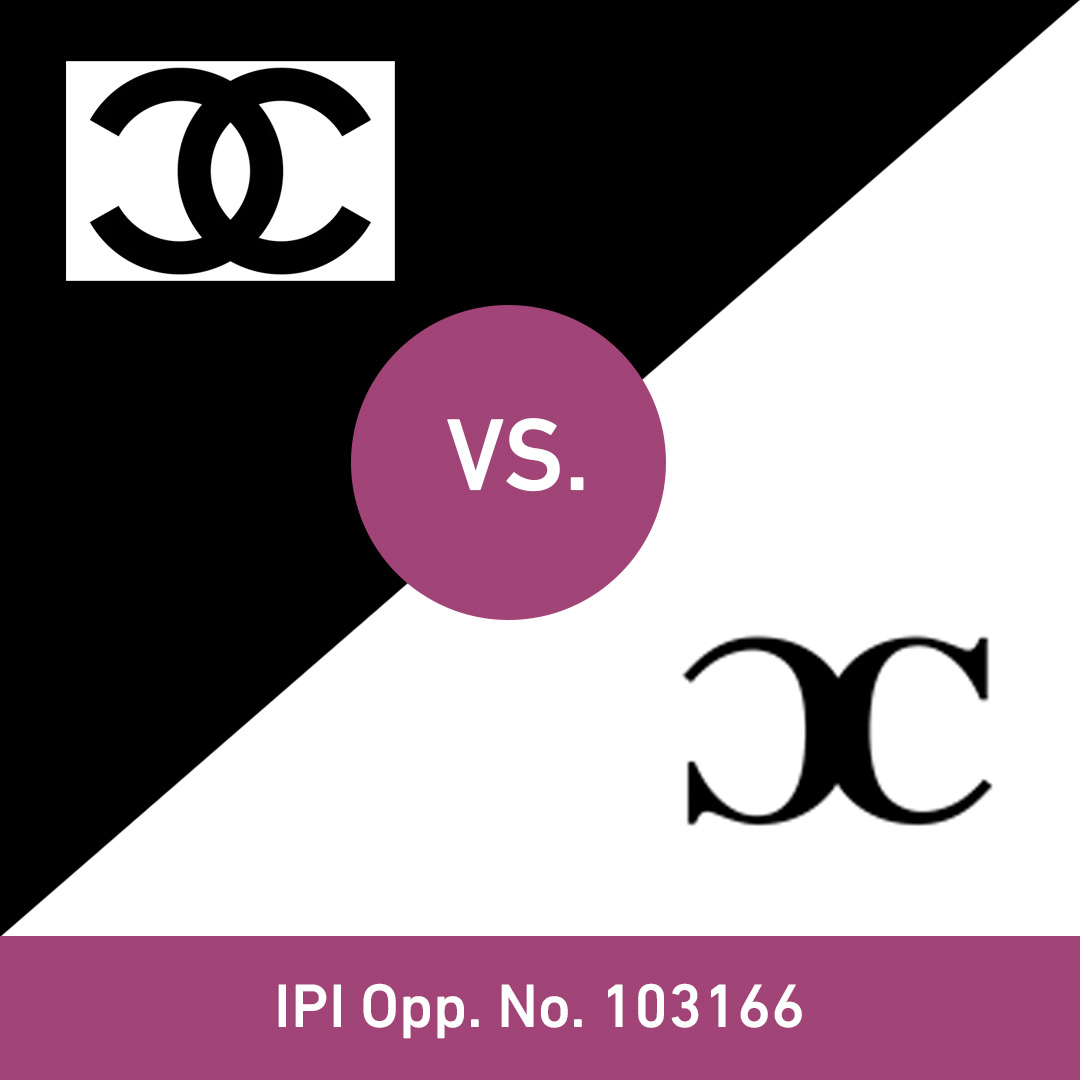
Trademarks often die slowly and silently
- Information
- First published 23 February 2024
A trademark is weakened not only by the use of identical and confusingly similar marks, but also by the registration of such marks.
Many owners of registered trademarks have never heard of trademark monitoring. Why should they? The trademark is registered and everything seems fine.
However, the trademark offices in most European countries (in Switzerland: the Swiss Federal Institute of Intellectual Property, IPI) and in many other countries only ensure that trademarks are registered, without checking whether identical or confusingly similar trademarks have already been registered. Trademark owners must therefore take the protection of their trademark rights into their own hands after successful registration.
Trademark owners can use trademark monitoring to be informed in good time of any disturbing trademarks that appear to be identical or confusingly similar and to take action against their registration by filing an opposition with the trademark offices. At the same time, they can often become aware of problematic use and consider taking legal action against it. This is an effective way of preventing the weakening and dilution of one's trademark rights.
The slides show recent examples of cases from the Swiss IPI where trademark owners have successfully defended themselves by filing oppositions.
- Details
- Written by Christian Ebner
- Hits: 384

- Information
- Subscribe
No patent protection in Europe for plants obtained by CRISPR and related technologies?
- Information
- First published 22 February 2024
The Members of the EU Parliament have agreed to adapt the rules for NGT plants (New Genomic Techniques, i.e. plants which have been obtained by genetic engineering tools, such as CRISPR/Cas). In their proposal, the MEPs aim to introduce a complete exclusion from patentability for all NGT plants. The intention behind seems to be to avoid legal uncertainties and dependencies for farmers and breeders.
While this intention is to some extent understandable (a farmer next door should be protected from inappropriate infringement claims), it may not be the right way to go.
Farmers/breeders are in many national patent law systems protected against unjust claims by clear legal limitations of the patent. In Switzerland for example, a patent cannot be enforced against a farmer if the protected biological material crosses into their original plants by coincidence (e.g. pollen carried by the wind). In addition, many national laws provide for a research privilege – or, for example in Switzerland, a specific breeder’s privilege which allows the use of patent protected biological material for the purposes of research and breeding of new varieties. A similar provision exists in the Agreement on the Unified Patent Court, which applies to Unitary Patents in many European countries. Therefore, access for breeders to develop new species is secured. Finally, farmers also benefit from the farmer’s privilege, as it is for example provided in Switzerland. When farmers legally acquire patent protected seeds, they can reproduce the product from material they have grown on their own farm for their own use (i.e. use today’s seed for next year’s harvest).
It is true that farmer’s rights, in particular the rights of small farmers, must be protected against unjust claims of patent infringement. Also research should be allowed to further advance technology. However, this can be achieved through legal exemptions of the patent’s effect.
Banning patents for such NGTs deprives researchers of the ability to protect their inventions and investments from their competitors.
An avocado plant which requires less water and can therefore help to save drinking water in European areas suffering from drought, is not developed in a few days. It requires significant research investments, long trials and safety assays. Companies will be much less inclined to commit their resources to such research programs if they cannot ensure that their investments are protected and competitors cannot act as mere copycats reaping their investment. Also European biotech start-ups developing such technologies will find it difficult to convince investors if their technology can simply be copied by larger players in the field.
A balance therefore needs to be struck between the understandable concerns of farmers and the interests of biotech research companies.
Picture by Eddie Pipocas via Unsplash
- Details
- Written by Alena Bach
- Hits: 340

- Information
- Subscribe
A quick glance at the patent class on capture and storage of greenhouse gases
- Information
- First published 21 February 2024
In preparation for a lecture on carbon capture at the ETH this spring semester, I took the opportunity to take a closer look at the new CPC patent class Y02C, which is dedicated to capture and storage of greenhouse gases. Here are some key findings:
- The country with the most patent families published in this class is China, followed by the United States.
- Notably, China has had more publications per year than the US since 2016, with an even more pronounced difference since 2021 (see chart below).
- In contrast, the number of patent families published by the EPO has remained more or less constant since 2013.
- The top assignees worldwide are Air Liquide, Mitsubishi Heavy Industries, Exxon Mobil Corp and Linde AG.
- The main applications are in the chemical sector, with around five times as many publications as in the mechanical engineering sector (there seems to be some room for improvement here).
The high number in the US is probably due to the US government's strong investment strategy in carbon capture and greater private funding. China is also set to play a prominent role in promoting green and low-carbon development, and the figures reflect the general trend of a large number of patent applications in China.
It remains to be seen how the patent landscape in Europe will evolve in the coming years.
- Details
- Written by Moritz Hönig
- Hits: 3302

- Information
- Subscribe
Challenges in licensing CRISPR gene editing
- Information
- First published 05 April 2022 - co-author(s): Alfred Köpf, Christian Ebner
CRISPR is considered to be "the biggest biotech discovery of the century” and was the subject of the Nobel Prize in Chemistry 2020. The technology has quickly become an indispensable tool for gene editing and has countless applications in various fields of research. It is no surprise that demand for licenses is high. By contrast, obtaining a license is often difficult. The article illustrates some of the questions and challenges associated with licensing CRISPR technology.
Who owns the patents for CRISPR gene editing?
Different researchers contributed to the CRISPR gene editing variations that exist today, including Jennifer Doudna and Emmanuelle Charpentier (who jointly received the Nobel Prize in Chemistry in 2020), as well Feng Zhang and George Church, among others. Owing to the diversity of contributors, important patents for CRISPR gene editing are held by different entities, including CRISPR Therapeutics, Intellia Therapeutics, ERS Genomics and Caribou Biosciences. Most of these entities were founded by the universities involved for the specific purpose of commercializing their CRIPSR patent portfolios. For an insightful overview and discussion of the different patent holders, see the article “Licensing the unlicensable”.
There are now more than 11’000 patent families on CRISPR and numerous legal disputes regarding rightful ownership of some of the early and broad patents are currently ongoing. Although the US Patent and Trademark Office has recently ruled in favor of the Broad Institute, the legal battle is likely to continue over the next few year.
Is a license required?
Whether or not a license is needed obviously depends on the specifics of a given case. However, for most applications, especially mainstream applications where CRISPR is used as a tool for gene editing, the answer is probably yes – in fact, multiple licenses may well be needed. The reason for this is that many applications fall under multiple patent rights, which means that a license is required in respect of all these patent rights. As an example, a state-of-the-art CRISPR gene editing application may fall under one or more of the broad early patents on CRISPR in general, as well as more recent patents relating, for example, to a specifically optimized version of the Cas protein.
Much to the dissatisfaction of prospective licensees, no comprehensive licensing pool exists for CRISPR gene editing which would include the major patent holders. This means that the patent portfolios of the different patent holders will have to be carefully analyzed to establish whether a given application falls under one of their patents.
One exception to the general obligation to obtain a license for a patent protected technology is provided by the broad Swiss research privilege. Similar exemptions do not necessarily exist in other countries, and if they exist, their extent may differ. In essence, the research privilege exempts research on the patented invention, including its potential uses, from the effect of a patent. As an example, no license may be required for investigations into the exact mechanism how the Cas9 protein effects double strand scissions. Perhaps commercially more relevant, the Swiss research privilege also extends to investigating potential uses of the patented invention. The extent and limitations of the research privilege is discussed in further detail in our article “Challenges and Opportunities in Licensing Biotechnological Research Tools” in “Life Science Recht”.
Challenges for startups and SME’s
The daunting reality for many prospective users of CRISPR gene editing technology is that they will have to wade through its complex licensing landscape in order to identify relevant patent rights, and then probably have to enter into licensing negotiations with different patent holders. This is particularly challenging for startup companies and SME’s for which the effort required is disproportionately high and, sometimes, prohibitively high. As such, it is worth considering national measures, such as the research privilege, which may significantly facilitate access to a patented technology like CRISPR gene editing under certain circumstances. For a more detailed analysis of the CRISPR licensing landscape and the legal measures available, see our article in “Life Science Recht”.
We gladly assist you in assessing the licensing needs for your specific application.
- Details
- Written by Adrian Fischbacher
- Hits: 2698

- Information
- Subscribe
New Trans-Atlantic Data Privacy Framework announced
- Information
- First published 26 March 2022
On 25 March 2022 the European Commission announced, that the EU and the US reached an agreement in principle for a new Trans-Atlantic Data Privacy Framework. The new framework would be a replacement for the the old EU-US Privacy Shield and the even older Safe Harbor framework, both of which had been declared invalid by ECJ rulings. The conclusion of a new agreement would again allow European companies to transfer personal data to the USA without having to deal with the restrictions in art. 45–49 GDPR.
The original two agreements between the EU and the US were declared invalid by the ECJ because US intelligence agencies may access data without EU data subjects having a legal remedy against this access. According to the information now available, the US government has agreed to limit access to data by US intelligence authorities to what is necessary and proportionate to protect national security. There is a risk that the ECJ will not consider these limitations to be sufficient, and that a new agreement will only lead to yet another Schrems decision (Schrems III?). However, this question will remain unanswered for some time until the ECJ issues a subsequent ruling. Until then, the future agreement – if it is definitely concluded – should bring significant relief for European companies in their dealings with US service providers. It is quite conceivable that Switzerland will conclude an analogous agreement with the US, as was already the case with the Swiss-US Privacy Shield. This would also allow Swiss companies to use US service providers relatively freely.
For more information, see the European Commission’s press release as well as the fact sheet.
Page 2 of 5